Lithium plating
This summary page is largely based on “Li plating as unwanted side reaction in commercial Li-ion cells – A review” (2018) by Waldmann, Hogg, and Wohlfahrt-Mehrens.
Lithium plating is a Cell capacity fade (i. e., a Cell degradation) mechanism. Instead of intercalating into an anode, Lithium forms a metal compound on the surface of the anode (below the SEI layer on the anode).
Lithium plating affects four key characteristics of cells: lifetime, low-temperature performance, how fast they can be charged, and safety.
A physical precondition of Lithium plating is that the standard electrode potential of Lithium intercalating into graphite anode (–2.84) is close to the potential of Lithium metal formation (–3.04).
The insertion potential of Lithium ions in graphite is in the range of 200 to 65 mV vs. Li/Li+ [metal deposition].
The potential of Lithium intercalation decreases, and, hence, the risk of Lithium plating increases when charging either 1) at low temperature (see below), 2) with a strong current, 3) at a high state-of-charge level (esp. overcharging). These lead to an increased concentration of Lithium close to the surface of the anode.
Lithium plating reduces the Lithium inventory in the electrolyte. It can also crack the SEI layer and grow into dendrites.
Lithium plating is one of the reasons why cells have Coulombic efficiency less than 1.0.
Anode overhang decreases the risk of Lithium plating
N/P ratio is the ratio of the areal reversible capacity of the negative electrode and positive electrode: Q_NE/Q_PE.
Liu et al. reported a lower ageing rate and lower impedance rise for an N/P ratio of 1.19 compared to cells with an N/P ratio of 1.06 [1].
Anode “overhang” prevents overcurrents at the edges. Overcurrents also lead to Lithium plating [2]:
Charging at low temperature leads to Lithium plating
This is due to Arrhenius law: at a lower temperature, Lithium ions are less likely to have high enough energy to cross the potential barrier (their energy is described by Maxwell—Boltzmann distribution), both on the anode-electrolyte interface (SEI), and within the graphite structure. The effect is that Lithium ions “crowd” close to the SEI, on both the anode and the electrolyte sides. On the anode side, this leads to a further increase of the diffusion voltage. On the electrolyte side, this “Lithium crowding” is the direct precursor for Lithium plating.
As an example, in a commercial cell, Lithium deposition (plating) starts at a charging C-rate ≥2C for 50 °C, and already at ≥0.5C at 12 °C [3]. (And at 1C charging rate at 25 °C: see the next section.)
More Lithium plating happens closer to the surface of a cell when charged at very low ambient temperature because the outer parts of the cell will be cooler:
Petzl et al. cycled 26650-type cylindrical cells at −22 °C with a charging rate of 1C and found a grey homogeneous film on the anode. They reported that the mass per area of the anode was higher on the outer part of the jelly roll, suggesting more Lithium plating near the cell housing.
Lithium deposition takes over SEI growth as the leading capacity fade mechanism at 1C rate and 25 °C
The capacity of the battery decreases the slowest when cycled at 1C rate and the temperature of about 25 °C [4]:
Small cells are less susceptible to Lithium plating than large cells
Small cells have more even internal temperatures and local state-of-charge (including due to smaller overall currents). Therefore, local Lithium deposition is less likely in smaller cells. (See cell size tradeoff.)
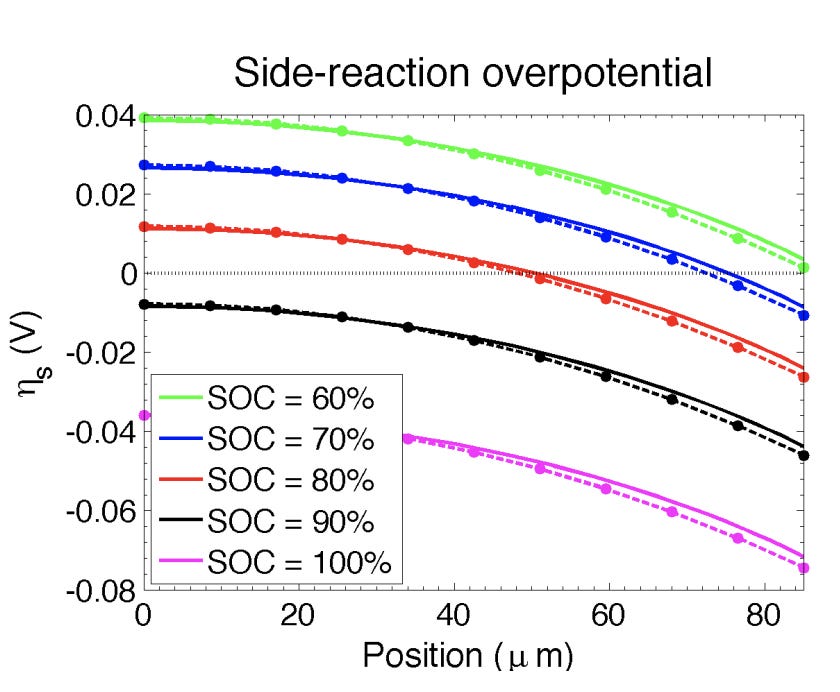
Similarly, a thinner anode is less susceptible to Lithium plating because of the smaller overall difference in Lithium concentration surrounding anode particles near the current collector (lowest concentration) and particles near the separator (highest concentration). The chart below models overpotential depending on the distance in the anode from the separator (x-axis):
Anode particles that are close to the separator also reach a high state-of-charge sooner than the particles which are close to the current collector. This will also contribute to uneven Lithium plating.
Cell capacity fade accelerates when Lithium deposition becomes irreversible
Cell capacity fade curves often have a recognisable knee:
Anseán et al. found that the Li deposition starts to get irreversible at the turning point of the sudden capacity drop [5].
Lithium dendrite growth has a positive feedback loop.
Lithium plating lowers the temperature of the onset of self-heating of cells
From a certain temperature, cells start to self-heat, e. g. at the rate of 0.02 °C/min. The onset temperature can be 100 °C for new cells but only 30 °C for cycled cells [6].
However, this assumes the cell cannot dissipate heat. An active cooling system in a battery can more than compensate for the self-heating of cells.
Lithium plating is responsible for the lower onset temperature because Lithium reacts with the electrolyte exothermically. Especially Lithium deposited in dendrites because dendrites have a large surface area. Furthermore, Lithium plating increases the self-heat rate [6].
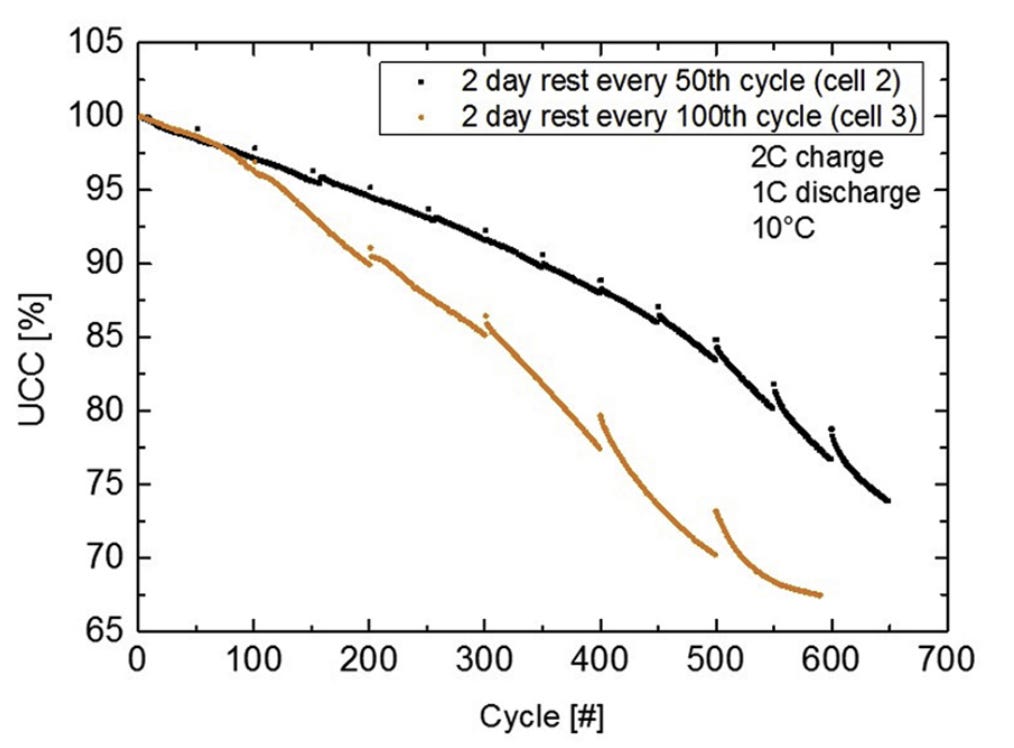
Frequent rest recovers capacity and prolongs a cell’s life because it recovers Lithium plating
Frequent rest recovers capacity and prolongs a cell's life because Lithium plating is a self-reinforcing process, therefore, preventing it early leads to compounding benefits.
This effect is pronounced when a cell is cycled non-stop:
See more details in the review of this paper.
"Bumpy" voltage relaxation indicates Lithium plating
According to the literature, a shoulder appears in the relaxation voltage curve if Lithium plating occurred in the charge step due to the coexistence of Lithium metal (0 V vs. Li/Li+) and graphite (0.08 V vs Li/Li+) on the anode side [7].
This paper also confirms that Lithium deposition starts to significantly occur at 1C rate and 25 °C (see a section above):
References
[1] Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode (2014)
[2] Two-Dimensional Modeling of Lithium Deposition during Cell Charging (2009)
[3] In-situ detection of lithium plating using high precision Coulometry (2015)
[4] Temperature dependent ageing mechanisms in Lithium-ion batteries – A Post-Mortem study (2014)
[6] Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries (2015)